Corrective actions (CA) and preventive actions (PA) are the main elements of operation and improvement quality management systems. They need to be planned on the basis of technical audits, inspections, expert assessments current and predicted state of the object and direct to eliminate the causes emergence inconsistencies and potential hazards.
The ISO 9001:2008 standard establishes two types of actions in case of nonconformities - correction and corrective actions. Correction - these are the actions taken to eliminate the nonconformity that has arisen . Corrective Actions are actions taken to eliminate the causes of nonconformity. Sources for design documentation: - complaints, - reports of inconsistencies, - instructions, acts. Preventive Actions – actions taken to prevent the occurrence inconsistencies. Sources for PD: - analysis of customer needs, - market analysis, - measurement of stakeholder interest.
Methods for carrying out CD and PD are selected in relation to the processes carried out by the enterprise and the products of these processes.
Development of a design documentation and design procedure is a necessary activity for organizations where equipment failures and incidents occur, various inconsistencies and potential dangers are present (the possibility of a failure occurring and its development into major accidents with negative consequences for human health, the environment, property, business, etc. .).
These inconsistencies are a consequence of imperfect technology and production organization, human errors, natural and climatic influences and other factors. Organization of work to eliminate and prevent nonconformities, due to high labor and financial costs, requires the effective use of resources necessary for the implementation of design documentation and PD, which must be adequate to nonconformities and commensurate with the significance of their impact, i.e. measurable and quantitatively related to the level of safety.
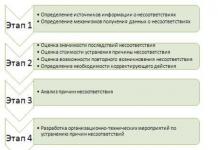
Usually, as a safety measure, they use the risk of an undesirable event, determined by the product of the probability of the event and the magnitude of the consequences. . Risk management includes a sequence of procedures:
Development of a management procedure corrective actions.

At the first stage of the procedure, it is necessary to identify sources of information about nonconformities and provide mechanisms for obtaining data on nonconformities.

At the second stage, the need for design documentation is determined based on an assessment of the significance of the consequences of the nonconformity, the cost of eliminating the cause of the nonconformity, and the possibility of its reoccurrence. At the third stage, an analysis of the causes of inconsistency is carried out using appropriate methods, for example: “brainstorming”, the precedent method, the “5 Whys” method, the method of constructing an “Ishikawa cause-and-effect diagram”, etc. At the fourth stage, organizational and technical measures are developed for eliminating the identified causes of nonconformities. These activities are usually aimed at improving the performance of the company as a whole. They can be carried out in relation to:

At the next stage, it is necessary to determine the rules for correlating certain inconsistencies with the levels of improvement in the organization's work. Corrective actions can address different levels of improvement - long-term, medium-term and operational. Long-term improvements typically involve all of an organization's processes, infrastructure, and production environment. Medium-term improvements affect individual processes or individual infrastructure elements. Operational improvements affect individual activities within processes.

The last stage of the procedure includes efficiency and effectiveness analysis actions taken. After the design work is completed, additional checks are carried out to confirm their effectiveness. The criterion for the effectiveness of design work is the absence of re-occurrence of discrepancies.
Procedure development management of preventive actions.
The procedure for preventive actions in terms of the composition of the stages is in many ways similar to the procedure for corrective actions,

but it differs fundamentally in the methods of obtaining initial information, to determine potential (possible) inconsistencies and in the nature of measures to eliminate the causes of potential inconsistencies. This procedure is more complex than the corrective action procedure, because requires the use of methods to identify and manage possible events. Such methods include risk management methods Therefore, elements of risk management are often included in the preventive action management procedure. Due to the fact that preventive actions are not so obvious, we can classify the types of actions mistakenly taken as warning:

Thus, to determine a preventive action, you need to pay attention to the following key signs:

The first step in this procedure is to identify sources of information about potential nonconformities. Sources of information are similar to sources of information for corrective actions

Preventive actions, like corrective actions, can address different levels of improvement in an organization - long-term improvements, medium-term and operational ones. Therefore, the next step will be to determine the rules for correlating potential nonconformities with levels of improvement in the organization's performance. If a potential non-conformity is identified and levels of improvement in the organization's performance are determined, a person is appointed who is responsible for conducting PD and meeting the requirements for it. The general procedure includes:

The effectiveness of preventive actions is assessed by the responsible person and, if necessary, by conducting special inspections and monitoring of facilities where potential nonconformities may occur. Performance criterion preventive action is the absence of actual occurrence of a potential nonconformity. A significant difference from the corrective action procedure arises in the details of the above general procedure, which are determined by the selected risk management methods.
Carrying out design documentation and PD according to well-thought-out and documented procedures containing methods for analyzing the causes of nonconformities, risk management, feasibility studies of the volume, measures to prevent nonconformities taking into account assessed risks - is one of the most important areas for improving the activities of enterprises. This will help improve the reliability and competitiveness of their products. Therefore, when implementation and development of QMS in accordance with ISO 9001:2008, an in-depth study of these methods is required with adaptation at a specific enterprise in the course of its development of mandatory documented procedures for conducting design documentation and PD.
15. Algorithm for selecting and monitoring suppliers. The procedure for comparative analysis of suppliers. The essence and task of the procurement department. STP “Procurement”.
Algorithm for selecting and monitoring suppliers.
The problem of supply quality management contains a number of aspects:
Clear specifications or drawings and purchase orders;
Approaches for selecting a supplier;
Supplier analysis (second party audit);
Determining the number of suppliers;
Interaction with suppliers;
Agreement on the distribution of responsibilities;
Ability to resolve controversial issues;
Organization of incoming supply control.
Approaches for selecting a supplier
One of the eight principles of the quality management system is the principle of “Mutually beneficial relationships with suppliers.” The choice of supplier should be made taking into account many factors, among which the main one is product quality. A predominant focus on the price of purchased materials or products increases the risk of producing low-quality products.
A more reliable and effective approach to selecting a supplier is based on analyzing information about the quality of work and products. This requires appropriate research and expense. With this approach, the supplier must allow representatives of the customer (consumer) to enter its enterprise.
Supplier audit
The supplier evaluation method includes:
Analysis of previous supply activities;
Establishing compliance of the supplier’s quality management system with the requirements of the standards;
Consumer control of product quality for compliance with standard requirements.
When assessing a supplier, consumer auditors consider the following aspects:
Management competence and commitment to quality;
Understanding of technical specifications and supply contracts;
The size of the enterprise and its production capacity;
Possible volume of supplies;
Quality management processes and methods;
Level of qualifications and training of employees;
Organization and effectiveness of quality assurance;
Reviews from other consumers;
Financial stability of the enterprise.
The audit is carried out before the contract is concluded.
The choice of supplier is based on:
Evaluation of a pilot batch of products;
Experience of previous work with similar suppliers;
Test results of previously supplied similar products by this supplier;
Known experience of other consumers;
Control at the enterprise and assessment of the functioning of the supplier’s quality system.
A) it allows you to choose the best supplier;
B) it provides feedback to correct supply disruptions.
2. Selection of suppliers using the prioritization method
Number of suppliers
Experience shows that the risk is minimal if there is only one supplier, but carefully selected.
The advantages of the "single supplier" principle are as follows:
Possibility of long-term cooperation, confidence in the future for both parties;
Cooperation helps in competition;
There is an opportunity to reduce costs for product control;
An increase in the volume of supply by one supplier, which leads to lower prices for the consumer.
Incoming product control
Incoming control is quality control of raw materials, materials, components or other products of the supplier before their use in the production of other products.
The purpose of incoming control when accepting products is to confirm that the purchased products have the specified quality. Control should ensure that the market is minimized and carried out as cost-effectively as possible.
There are three types of incoming control:
1. One hundred percent control of all supplied products is very expensive, time-consuming and not always feasible. Such control is used in single or small-scale production.
2. During sampling control, a relatively small number of product units from the entire population are checked. The sample size is calculated based on the statistical laws of probability theory.
Such control is most suitable for serial and mass production. However, with such control there is a certain risk of missing a certain amount of non-conforming products. Therefore, the acceptable level of defects or rejects is established and controlled by the consumer.
Sampling based on marginal quality is often used, i.e. Share or permissible percentage of defects.
Depending on the number of samples selected for control, the following types of control plans are distinguished:
A one-stage sampling plan is characterized by the fact that the decision to accept a batch of products is made based on the results of control of only one sample.
With a two-stage sampling plan, the decision to accept a batch of products is made based on the results of control of no more than two samples, and the need to select a second sample depends on the result of control of the first.
With a multi-stage sampling plan, the decision is made based on the results of monitoring several samples, the maximum number of which is set in advance, and the need to select a subsequent sample depends on the results of monitoring previous samples.
A sequential sampling plan is characterized by the fact that the decision to accept a batch of products is made based on the results of several samples, the maximum number of which is not established in advance, and the need to select a subsequent sample depends on the results of control of previous samples.
The control level is a characteristic of the control plan that links the sample size with the volume of the product batch. GOST establishes seven levels of control:
General and special
The most commonly used level is II – the general level of control.
3. Random sampling.
The procedure for comparative analysis of suppliers.
This is an expert method, it allows you to compare suppliers according to various criteria that have units of measurement or are qualitative and require assessment in points. The number of criteria and suppliers being compared is not limited.
The Priority Setting Method is an expert method; to compare options, it is important that
The experts’ judgments were quite adequate, for which a group of experts is first assessed using one of the methods. The number of experts in the group is no more than 7 people.
3.2. Main stages of the MCI
The MRP method involves several stages:
1) selection of objects for comparison;
Objects for comparison must be homogeneous, i.e. Belong to the same class, or standard size, or to the same type, etc.
2) selection of criteria for comparison;
The criteria can be quantitative, representing parameters that have units of measurement, for example, the highest developed speed of a car (km/h). The criteria can be qualitative, for example design, convenience, etc. In this case, experts must develop a scale for comparing options according to this criterion, such as in figure skating.
If the criterion is an organoleptic indicator, for example, taste, then experts evaluate the options, for example, on a 5-point scale individually, and then the result for each option is averaged.
For example, options for school notebooks with 18 sheets from different manufacturers are compared. Significant indicators will be:
Surface roughness, microns;
Paper density (kg/m2);
Design (in points on a scale);
Cover quality (in points on a scale);
Price, rub).
3) compiling a matrix of initial data;
Matrix of initial data in the form of a table.
On the left is a column of numbered criteria for comparison. The central part of the matrix contains the values of the criteria for each of the options being compared.
This example compares product suppliers.
Since prices change, experts have put down a conditional price ratio for each option.
The shelf life has units of measurement and is based on the experienced and declared data of the manufacturers.
4) compiling matrices of paired comparisons to determine the ranks of options for each criterion;
Matrices of paired comparisons of options are compiled for each criterion, as a result of which the preference ranks of options according to the criteria are determined.
5) calculation of coefficients for assessing the signs of relationships between criteria;
6) drawing up a matrix for assessing the importance of criteria;
This is the most critical stage of calculations, since at this stage much depends on the adequacy of expert judgments, and it is at this stage that some error in the result is introduced.
To compare criteria by importance, already known relationship signs are used, but the symbol “>” here means “better”, the symbol “<» - хуже, символ «=» - равнозначно.
7) drawing up a final matrix to determine relative priorities.
At the final stage of the analysis, a matrix of relative priorities is built on the basis of data from previously constructed matrices.
The essence and task of the procurement department
The purpose of the purchasing department is reliable and high-quality provision of the company’s production departments with the material resources necessary to fulfill the given production schedule nia.
Relations with suppliers should be based on the following principles:
· treat suppliers in the same way as clients of the company;
· do not forget to actually demonstrate a commonality of interests;
· introduce the supplier to your tasks and be aware of his business operations;
· show readiness to help if problems arise with the supplier;
· comply with the obligations assumed;
· take into account the interests of the supplier in business practice.
Purchasing department tasks:
· determination of the need for material supplies;
· procurement market research;
· selection of suppliers;
· implementation of procurement;
· supply control;
· preparation of procurement budget;
· coordination and systemic relationship between procurement and production, sales, warehousing and
· transportation, as well as with suppliers.
The central place in purchasing logistics is occupied by the problem of effective coordination of processes for promoting material flows. Its solution involves the creation of a flexible system of centralized operational management, regulation and control of the provision process
STP Procurement
Enterprise standards (STP) are established for objects that are used only at a specific enterprise. STPs are approved by the management of the enterprise.
For the Purchasing process, 1 standard can be developed at the enterprise, or it can be divided into several components (“Purchasing. Evaluation and selection of suppliers”, “Purchasing. The procedure for approving samples from external suppliers for launch into production.”, “Purchasing. Incoming inspection of materials and components", "Purchases. Purchase of materials and components for the production of products." The composition of the STP Procurement can be seen using the example of the STP "Purchases. Evaluation and selection of suppliers":
1. General Provisions
1.1. Result of the process
1.2. Process owner
1.3. Process executors
1.4. Process documentation
2. Process diagram
3. Interaction with other processes and the external environment
4. Organization of process execution
4.1. Subprocess “A6.4.1 Preparation of a draft contract with a supplier or its extension”
4.2. Subprocess “A6.4.2 Legal registration of the agreement (TA)”
4.3. Subprocess “A6.4.3 Agreement agreement with interested parties”
4.4. Subprocess “A6.4.4 Agreement agreement with the Director of Economics”
4.5. Subprocess “A6.4.5 Conclusion of an agreement (TA)”
16. Motivation and its role in QMS. Types and methods of motivation in QMS.
In management company, personnel motivation is encouraging employees to actively work to ensure the required quality of products. Motivation is based on the principle of opportunities for employees to realize personal goals through a conscientious attitude towards work. Without the interest of employees, any plans for improving quality will remain only on paper. Initially, motivation came down to the carrot and stick method; Taylor improved this method by proposing to pay labor in proportion to the volume of output, as a result of which labor productivity increased significantly. But as the welfare of workers grew, the use of this method became insufficient. With the development of psychology and sociology, substantive theories of motivation developed, as well as procedural theories of motivation, taking into account the motives of people's behavior in the workplace. For motivation according to Maslow's needs in 1940. proposed to use a hierarchy of needs, which is a pyramid, at the base of which there are primary needs (physiological), at the second level - the needs for safety and security, then in ascending order, he placed social needs, the needs for recognition and self-expression.
Motivation of employees may involve the use of different methods of material and non-material motivation. Among them are increased salaries and bonuses, protection from deterioration of financial situation, improved working conditions, social contacts, distribution of company shares among employees, conferment of honorary titles, increased status, promotion, provision of more interesting work, the opportunity to receive education and engage in interesting activities. , free distribution of working hours, etc.
Currently in Russia, the basis of motivation is the level of salary and the satisfaction of social needs. An important feature of the work on motivating personnel at an enterprise is the need for close interaction with trade unions and the legal service.
Considering the importance of the quality of manufactured goods for the economy as a whole, the production of high-quality products is also stimulated at the state level by awarding quality awards to enterprises (since 1997 - the Russian Quality Prize is awarded: For the achievement of significant results by an organization in ensuring the safety and quality of products and services; For the implementation of highly effective quality management methods.)
Corrective and preventive actions. Basic provisions.
PREFACE
The standard is intended for organizing corrective and preventive work aimed at continuously improving the effectiveness of the quality management system.
The standard was developed by the BUSK service;
During its development, the requirements of ISO/TS 16949 section 8.5 “Improvement” were taken into account.
Preface
- Application area
- Normative references
- Definitions
- Notations and abbreviations
- General provisions
- Corrective Actions
- Preventive actions.
- Continuous improvement
- Levels of taking corrective, preventive and continuous improvement actions
- Organization of corrective and preventive actions in production
- Analysis of the causes of inconsistencies
- Development and implementation of corrective and preventive actions
- Assessing the effectiveness of corrective and preventive actions
- APPENDIX A (recommended) Workplace Scheme
- APPENDIX 5 (recommended) Corrective and preventive action plan form
1 area of use
1.1 This standard establishes the basic rules and procedures to be followed at the enterprise when organizing and carrying out corrective and preventive actions, as well as assessing their effectiveness.
1.2 The standard applies to all departments within the scope of the QMS.
1.3 As part of this activity, the enterprise standard establishes the responsibilities and interaction of relevant departments and officials in the implementation of the rules and procedures provided for therein.
2 Normative references
When developing this standard, the requirements and recommendations of the following regulatory documentation were taken into account:
- Quality Management Systems. Special requirements for the application of the ISO 90012008 8 standard in the automotive industry and organizations supplying relevant spare parts.
- Quality management systems. Fundamentals and vocabulary, Instructions. Quality Management System. Management Responsibility. Analysis of the QMS by management.
- Instructions. Quality Management System. Management Responsibility. Analysis of the QMS by Management.
- Enterprise standard. Quality system. Quality planning.
- Enterprise standard. Quality system. Process development management
- Enterprise standard. Quality Management System. Documentation and data management. Basic provisions.
- Enterprise standard. Quality system. Design documentation management
- Enterprise standard. Quality system. Technological documentation management.
- Enterprise standard. Quality system. The procedure for receiving purchased products into the enterprise’s warehouses, carrying out their incoming inspection, storage and release into production
- Enterprise standard. Quality Management System. Organization of operation of scheduled preventative maintenance and repair of equipment.
- Enterprise standard. Quality Management System. Organization of operation of scheduled preventive maintenance and repair of electrical power equipment
- Enterprise standard. Quality system. Providing production with technological equipment and measuring instruments.
- Enterprise standard. Quality system. Organization of control of technological discipline in production.
- Enterprise standard. Quality Management System. Control and testing. Basic provisions.
- Enterprise standard. Quality system. Periodic and standard testing of products and their components.
- Organization of the event, evaluation of results. Enterprise standard. Quality system. Management of control, measuring and testing equipment. Basic provisions.
- Enterprise standard. Quality Management System. Management of non-conforming products. Basic provisions.
- Regulations on the decision-making commission.
- Instructions. Carrying out loading, unloading and transportation operations.
- Enterprise standard. Quality Management System. Internal audits. Basic provisions.
- Enterprise standard. Quality system. Personnel training. Basic provisions.
- Enterprise standard. Quality Management System. Maintenance. Basic provisions.
- Enterprise standard. Quality system. Statistical methods. Basic provisions.
3 Definitions
This enterprise standard uses terms with corresponding definitions:
- Corrective Action— action taken to eliminate the cause of a detected nonconformity or other undesirable situation.
- Preventive action— action taken to eliminate the cause of a potential nonconformity or other potentially undesirable situation.
- Continuous improvement— repeated activities to increase the ability to meet requirements.
- Inconsistency- failure to comply with the requirement.
- Requirement- a need or expectation that is stated, generally assumed, or required.
- Efficiency— the degree of implementation of planned activities and achievement of planned results.
- Efficiency— Relationship between the achieved result and the resources used.
- Production factors (resources)— these may include: raw materials, workpieces, test instruments, equipment, fixtures, tools, fixtures, personnel, documentation, control and testing tools, software.
4 Symbols and abbreviations
- BUSK— quality system management bureau
- DI- job description
- KD- design documentation
- CI- components
- ND- normative documents
- Quality Control Department— technical control department
- RI- working instructions
- SI- measuring
- QMS- Quality Management System
- STP- enterprise standard
- ONE HUNDRED— technological equipment
- TD— technological documentation
- TB- technology bureau
- TK- technical task
- TI— technological instructions
- TP- technological process
- TI— technical instructions
- TB- technology bureau
5 General provisions
5.1 Corrective actions
5.1.1 The purpose of applying corrective actions is to eliminate the causes of nonconformities that have occurred to prevent their reoccurrence.
5.1.2 Corrective actions are carried out in all QMS processes (including control processes, main and supporting processes) when inconsistencies are detected.
Responsibility for applying corrective actions lies with the heads of processes and departments.
5.1.3 Sources of information about nonconformities for determining corrective actions are:
- complaints, claims from consumers (external and internal);
- reports on inconsistencies (including based on the results of internal and external audits);
- output data for assessing the achievement of established and desired goals;
- process performance assessment outputs;
- results of analysis of the QMS and processes by management;
- self-assessment results.
5.1.4 To effectively identify the causes of inconsistencies, it is advisable to use a wide range of statistical methods (such as Pareto, Ishikawa diagrams, scatter diagrams, etc.).
Determination of the causes of nonconformities should be carried out by an individual or a committee appointed to develop corrective actions.
5.1.5 Before corrective action is taken, the significance of the problem must be assessed in terms of the potential impact on aspects such as operating costs, cost of nonconformity, product performance, reliability, safety, and customer and other interested party satisfaction.
Corrective action investments should be prioritized based on the likely consequences of the problem being addressed.
5.1.6 The process manager organizes the development and coordination of corrective actions with co-executing units and monitors their implementation.
The results achieved by corrective actions must be recorded through records.
5.1.7 Analysis of the effectiveness of the corrective actions taken is carried out in subsequent periods of time by comparing the achieved indicators with the indicators of previous periods.
5.2 Preventive actions
5.2.1 The purpose of applying preventive actions is to eliminate the causes of potential nonconformities and prevent undesirable situations.
5.2.2 Preventive actions to reduce possible losses should be applied in core and supporting processes, as well as in control processes to increase stakeholder satisfaction.
Responsibility for the application of preventive actions lies with the heads of processes and departments.
5.2.3 Reducing losses in an organization through the use of preventive actions must be planned.
To achieve effective application, planning preventive actions must be systematic.
5.2.4 Input data for identifying potential nonconformities can be obtained through:
- using tools (means, methods) for risk analysis (for example, such as analysis of the nature and consequences of failure - PMEA);
- analysis of consumer needs and expectations, market analysis;
- measuring stakeholder satisfaction;
- assessment of trends in process changes;
- self-assessment results.
5.2.5 Detailed and systematic analysis of processes provides information on potential nonconformities to develop loss prevention plans and identify priorities for improvement related to the processes under consideration.
5.2.6 After identifying a potential nonconformity, further activities to determine the causes of the nonconformity and take preventive actions are carried out similarly to corrective actions.
5.3 Continuous improvement
5.3.1 Activities aimed at continuous improvement of process performance, initiated by the manager or process participants, are preferable to eliminating nonconformities through corrective actions.
Continuous improvement activities reduce the likelihood of nonconformities and minimize waste.
The task of managers at all levels is to constantly improve the processes assigned to them.
5.3.2 It is necessary to strive to improve the organization’s processes, in terms of:
- increasing effectiveness (degree of fulfillment of requirements);
- increasing efficiency (for example, through resource optimization);
- streamlining impacts on the process (for example, changes in procedures and regulations);
- applying best practices, best practices, and not waiting for a problem to arise to identify opportunities for improvement.
5.3.3 Improvements can range from incremental daily steps to strategic breakthrough projects.
5.3.4 The basis of continuous improvement is the active search for opportunities to improve the performance of processes, activities and product characteristics.
This can be achieved through activities such as:
- determination of enterprise strategy and policy;
- management leadership for improvement;
- setting goals for all levels of the organization;
- comparison with competitors' achievements and best practices;
- involving employees in improvement activities (kaizen proposals);
- teamwork within projects;
- continuous training and professional development;
- recognition and reward for achieving improvements.
5.4 Levels of taking corrective, preventive and continuous improvement actions
5.4.1 Analysis of the causes of nonconformities, adoption and development of corrective and preventive actions, as well as continuous improvement activities can be carried out at different levels of enterprise management.
1) Operational measures at the factory level:
- meetings of directors by area (Meeting Matrix).
2) Prospective activities at the factory level:
- technical re-equipment plan;
- quality plan.
3) Operational meetings in departments:
- quality days in departments;
- workshops (Meeting Matrix).
4) Planned work to ensure constant compliance of products, processes and QMS with the requirements of design documentation, TD, ND QMS.
6 Organization of corrective and preventive actions in production
6.1 Inconsistencies in products, processes, QMS are identified and recorded in the process of daily and periodic supervision of production factors by production personnel, technologists and the department’s quality control department.
6.2 Inconsistencies in products, processes and QMS can be identified:
- during entrance control;
- according to data from the contractor during production and quality control of products;
- production foreman (foreman), or technologist with systematic supervision of the product manufacturing process;
- when controlling technological discipline;
- during intermediate and final quality control inspection;
- when analyzing non-conforming products of the manufacturing workshop by the consumer workshop;
- when analyzing defects during periodic and standard tests, carried out both in-house and with the involvement of third-party organizations;
- when analyzing product returns by consumers;
- when conducting internal and external audits, analysis of the QMS by management;
6.3 If a nonconformity is detected in a product, process, or QMS, the person who discovered the nonconformity promptly brings the information to the manager and records the nonconformity in the Problem Solving Log or in the Daily Supervision Log.
The person who discovers the discrepancy must indicate his position and surname in the appropriate column when registering it.
6.4 The head of the relevant department, having received information about the nonconformity (problem), analyzes the causes of the nonconformity and develops corrective and preventive actions to eliminate the causes of nonconformities in products, monitors their implementation and evaluates the effectiveness.
6.5 If the reason for the nonconformity is:
- consists in violation or failure to follow the sequence
- operations, methods and technological regimes;
- known and reflected in the “Classifier of inconsistencies”;
- is clear without the need for a comprehensive analysis, then in such cases the technologist, foreman, or foreman quickly develops and monitors the implementation of corrective and preventive actions to eliminate nonconformities.
6.6 Otherwise - if:
the measures taken did not allow the nonconformity to be eliminated or the cause of its occurrence to be clearly determined;
In the “Classifier of Nonconformities” there is no nonconformity being analyzed at the moment, then the foreman (technologist, foreman) needs to conduct a comprehensive analysis of the state of processes and all production factors, with the involvement of specialists from other departments responsible for supervising the state of production factors and upon completion of the analysis proceed to the development of corrective and preventive actions.
6.7 The foreman, foreman, and technologist records in the Daily Supervision Journal, Problem Solving Journal the causes of nonconformity, developed corrective and preventive actions, performers and deadlines.
6.8 An obligatory stage in carrying out corrective and preventive measures is the assessment of their effectiveness. If the measures taken do not eliminate the inconsistencies or do not eliminate the causes of their occurrence, the cycle of work to analyze these causes, develop measures and implement them is repeated.
6.9 If it is impossible to carry out any stage of work on our own, information about the non-compliance is reported to the management of the workshop (division).
6.10 The workshop management organizes work to analyze the causes of inconsistencies or develop measures with the involvement of specialists from departments whose competence is to solve these problems (shop, factory commissions).
7 Analysis of the causes of inconsistencies
7.1 All nonconformities related to products, processes and QMS must be analyzed by the appropriate officials in order to determine the causes of their occurrence. When analyzing the causes of nonconformities, you should:
- establish the possible influence of all actual and potential production factors;
- determine the location of the nonconformity, the form of manifestation and possible consequences.
7.2 Having received information about non-compliance, a comprehensive analysis of the state of all production factors operating in the workplace is carried out. In this case, the “Workplace Provision Scheme” is used (Appendix A).
7.3 If necessary, specialists from other departments responsible for monitoring the state of production factors are involved in the analysis.
A decision-making commission is convened, shop, inter-shop or factory.
The commission must establish the relationship between the nonconformity and its cause, taking into account the possible influence of all actual and potential production factors on the occurrence of nonconformities.
7.4 When analyzing the possible influence of production factors on the occurrence of nonconformities, the following is assessed:
- the condition of the initial raw materials, materials and clinical trials - the accompanying documentation is checked with a note on the results of incoming inspection, storage conditions, preparation before use in production;
- compliance with the RD for the presence of the latest changes in design documentation and technical documentation, the impact of changes on quality, the contractor’s knowledge of the changes made to the RD, as well as the clarity of technological instructions;
- compliance with technological discipline requirements;
- availability, sufficiency, and serviceability of service stations (wear, repair or replacement of equipment, tools, frequency of verification);
- equipment condition - in terms of downtime of process equipment, which has a destabilizing effect on product quality - the presence and cause of downtime, implementation of the schedule for preventive maintenance and repair of equipment should be analyzed;
- compliance with TP parameters, first of all, Critical ones;
- availability of packaging and perfection of transportation methods;
- turnover in the workplace;
- the contractor’s knowledge of the technology requirements, work instructions, sufficiency of the contractor’s qualifications;
- completeness of work instructions;
- completeness and compliance with the requirements of QMS documents;
- compliance with industrial safety and environmental requirements (air temperature, noise level, dust, etc.).
The result of the analysis should be:
determining the cause of the nonconformity;
development of measures to eliminate and further prevent the recurrence of nonconformities (corrective and preventive actions).
The period within which the analysis must be carried out is no more than 3 days.
The result is the determination of the cause of the nonconformity, the development of measures to eliminate and further prevent the recurrence of nonconformity.
The documented analysis protocol (8-D) should include instructions for corrective and preventive actions.
At the same time, in the “Problem Solving” and “Daily Supervision” Journals, a link to this protocol is given in the corresponding columns.
8 Development and implementation of corrective and preventive actions
8.1 The purpose of developing measures to eliminate nonconformities is to determine:
- corrective actions - to promptly eliminate the specific cause of nonconformities;
- preventive actions - to eliminate the causes of potential nonconformities, i.e. inconsistencies that may arise.
8.2 The process of taking measures to eliminate the causes of nonconformities involves planning (organizing), implementing and reporting on the implementation of activities.
8.3 Responsible for planning (organizing), implementing and reporting on the implementation of activities is the head (representative - technologist, foreman, quality engineer) of the unit in whose work inconsistencies were discovered.
8.4 Corrective and preventive measures are developed by the persons specified in clause 8.3 based on the established causes of nonconformity.
8.5 When developing activities, the following recommendations can be used:
- change methods of manufacturing products, equipment, devices;
- change methods of storage and transportation of products;
- reject, return and replace purchased materials, raw materials and CI;
- replace the supplier of purchased materials, raw materials and CI;
- develop, together with the supplier, programs to ensure and improve the quality of purchased materials, raw materials and CT;
- refine and regulate the manufacturing process of products;
- change control and testing methods;
- replace equipment, measuring and testing instruments, change production conditions;
- improve procedures for setting up equipment and adjusting processes;
- train and certify personnel;
- adjust QMS procedures and documents;
- change the wage system, etc.
8.6 The persons specified in clause 8.3 determine the content of activities, performers, deadlines for implementation and control the completeness and quality of implementation of activities; if necessary, specialists from other departments are involved.
8.7 Developed corrective and preventive actions are recorded in the daily supervision log, or problem solving log.
8.8 If it is impossible to fulfill the requirements of clause 8.7 (due to the large volume), the developed measures are documented in the form of a separate corrective and preventive action plan (Appendix 5) or Protocol 8-D. In this case, journals make reference to this document.
8.9 If there are co-executors from other departments, the developed activities are coordinated with them, duplicated and sent to them against signature.
8.10 Constant monitoring of the implementation of developed corrective and preventive actions is carried out by the responsible person specified in clause 8.3.
8.11 Periodic control (at least once a month) over the maintenance of the daily supervision journal (Problem Solution Journal) and the implementation of the developed measures is carried out by the workshop technologist (defect analysis engineer).
8.12 Corrective and preventive actions can be both operational and prospective.
8.13 Operational activities and the timing of their implementation after development are monitored:
- foreman - in the course of production activities;
- foreman, workshop technologist, workshop manager - at operational meetings, workshop “Quality Days”.
8.14. Information on the implementation of activities is documented in monthly QCD quality reports.
8.15 Prospective activities should be included in:
- "Quality Improvement Plan";
- Technical re-equipment plan.
8.16 Particular attention should be paid to preventive measures, since they make it possible to prevent and prevent the occurrence of possible inconsistencies.
8.17 Preventive actions should include improvement of QMS processes, methods and procedures. If necessary, based on the results of preventive actions, changes are made to the ND, TI, RI, DI, and other QMS documents.
8.18 Supervision over the development of corrective and preventive measures is carried out by the head of the quality control department (quality director).
9 Assessing the effectiveness of corrective and preventive actions
9.1 Timely implementation of corrective and preventive actions ensures that the enterprise reduces costs caused by the occurrence of inconsistencies in products, processes and QMS.
9.2 The effectiveness of activities is assessed based on the results of observations of the state of quality of products, processes and QMS after their implementation.
9.3 An event after which this discrepancy does not appear can be considered effective. This can be confirmed by data that the product defect, non-conformity in the process or QMS, for which corrective and preventive actions were carried out, have been eliminated and are not repeated.
9.4 Evaluation of the effectiveness of activities is carried out:
- foreman (foreman) - after carrying out activities and during periodic supervision;
- by employees of the quality control and safety department of the workshop - when monitoring products and technological discipline;
- GDT - when monitoring technological discipline;
- by the head of the workshop - during periodic monitoring and analysis of the department’s activities, with the obligatory communication of information to the relevant persons (foreman, technologist, etc.).
9.5 If the planned event is recognized as effective, the official who developed and monitors the quality and completeness of the implementation of the activities makes appropriate notes in a document containing corrective and preventive actions, for example:
- B Daily Supervision Journal, Problem Solving Journal;
- In terms of corrective and preventive actions;
- in a protocol or other document.
9.6 If the action to eliminate inconsistencies in the product, process or QMS turns out to be ineffective, the foreman (foreman), technologist informs the shop manager about the lack of results, indicating the reasons, and re-analyzes the inconsistencies and develops corrective and preventive actions in accordance with Section 8.
9.7 Information on the effectiveness of corrective and preventive actions is reviewed at operational meetings and Quality Days.
Purpose of the lecture:
- show the nature of actions that change the system - both corrective and preventive,
- show (with an example) possible preventive actions,
- analyze typical mistakes.
This topic is, as they say, one of the “eternal” ones, like a fire, either dying out or flaring up with renewed vigor.
I have already devoted one lecture to improving the system in the form of corrective actions, now it’s time to deal with warning ones.
But I will start talking about them with the same CDs - for two reasons: firstly, repetition is always useful, and secondly, a better understanding of corrective actions serves as a certain guarantee of a better understanding of preventive ones.
As practice shows, very often two different actions are mixed up. ISO 9000 calls one a “correction” and the other a “corrective action.”
It is very important to understand the mechanisms for improving the system in detail and, as they say, to understand it completely.
So, some activity led to a result. At the control stage, this result was compared with the given norm and it was found that it did not correspond to it. Our quality management system, designed to ensure compliance with requirements, must include actions to correct nonconformities. In this case, we must make a correction, i.e. try to correct the result so that it meets the requirements.
I draw special attention to the main feature of correction: it is aimed directly at the result of the activity, its goal is to bring this result into compliance with the requirements.
Example No. 1. We released the drawing, it did not pass the standard control - it does not comply with the ESKD. We took and made corrections to the drawing to remove inconsistencies. Thus, a correction was made.
Example No. 2. We handed over to the customer a project whose explanatory note did not contain sections stipulated by the contract. We have finalized the project by adding the required information. Those. We have corrected the project.
But, unfortunately, performing a correction only affects a specific product and does not provide any guarantee that the next time the same activity will produce a result that meets the requirements. Those. In order to improve the quality of our products, we must analyze the activities and find the reason in them that causes discrepancies. And eliminate it.
Actions that eliminate the cause of nonconformities are corrective actions.
Particular attention should be paid to the fact that design control is not aimed at correcting the result, but at changing activities in order to exclude from it what leads to inconsistencies.
And what else is extremely important when performing CD.
1 . The design documentation must eliminate the cause of inconsistencies. This means that having completed the CD, we should no longer receive inconsistencies for this reason.
Example No. 1. Training is often referred to as CD. For example, it is said that the cause of the nonconformity was lack of competence. We need training to improve it.
This is fundamentally wrong! What's wrong here?
Firstly, the cause is incorrectly identified. The performer's competence is not part of the activity, so increasing competence (through training) will not change our activity in any way. It is easy to see that as soon as the analyzed activity is performed by an untrained employee, we will immediately again get a discrepancy between the result and the requirements. Those. the reason has not been eliminated by training.
Secondly, the real reason for the discrepancy, which lies in the activity itself, in its organization, is that the work was entrusted to an incompetent employee. That. the corrective action in this case should be such as to change the organization of our activities, introduce into it a mechanism that would ensure compliance with the complexity of the work and the competence of the performer.
2. CD should not be undertaken based on one or two discrepancies. This leads to a high risk of misidentifying the cause and making incorrect changes to the system. CD should eliminate systemic causes, i.e. those that appear constantly. And for this we need statistics.
Well, perhaps, let’s finish here about corrective actions and return to our sheep, that is, warning ones.
Practice shows that there are certain difficulties in understanding what “preventive actions” are. If with CD it’s even more or less clear, then with PD...
What is a “potential non-conformity”? How does it differ from those inconsistencies for which CA is undertaken to eliminate the causes?
The ISO 9000 dictionary provides answers to these questions; you just need to be able to read, as they say.
The articles defining PD and CP (3.6.4 and 3.6.5) refer to “potential nonconformity” (PD) and “detected nonconformity” (DC). From this we can conclude that a “potential non-conformity” is an “UN-detected non-conformity”, i.e. one that has not yet been identified. Those. we can say that this is only the risk of nonconformity, and not the nonconformity itself (if the risk is interpreted as an opportunity).
But if there is no discrepancy, then how can you eliminate the reasons that may cause it?!
Answer: based on a) past experience and b) the experience of others.
Let us, so that the reasoning is not unfounded, let us take for analysis the situation described by Maya Aktanova in one of the forum threads: http://quality.eup.ru/forum/viewtopic.php?t=7792: “If a discrepancy is identified in one department, If the Policy has not been communicated to the personnel, is it a preventive action to familiarize the personnel of all departments with the Policy? If a complaint is received for one type of product, but there is a possibility of receiving the same complaints for other types of products, then eliminating the causes of these complaints for all types of products is a preventive action to eliminate the causes of potential nonconformities?
First, let's look at the situation with quality policy.
Firstly, it is obvious that it makes no sense to talk about preventive actions here, because the discrepancy has already been detected (identified), it is not potential. And if we take action to eliminate the cause of this discrepancy, then it - this action - will be corrective.
But can we consider “making staff aware of the policy” a corrective action in this case? To do this, we must understand whether it eliminates the cause of the detected discrepancy. So, we conducted an acquaintance. Does this guarantee that we will not get the same non-conformity again at the next audit? Answer: no, it does not guarantee. During this time, new employees will arrive, who will be “unfamiliarized.”
Then what should the CD consist of in this case? Let us remember that the design committee must change the system, make changes to the procedure. What is missing in our system, what mechanisms are missing? It is clear that we do not have a mechanism that would ensure that all employees are familiar with the policy (and also identify the level of understanding of it and allow the policy to be adjusted if it does not meet the requirements). This means that our system must provide:
- familiarization of employees with the policy at the time of its entry into force,
- identifying the degree of understanding of the policy by employees,
- carrying out work to explain the policy to employees,
- collecting data on the necessary adjustments to the policy related to misunderstanding of certain provisions (i.e. if people do not understand the policy and it is impossible to explain to them, you should consider changing the policy to make it more understandable),
- familiarizing new employees with the policy.
If our system does not have any of this, then the introduction of the missing mechanisms will be the desired CA.
Now mentally simulate a situation where all of the above is fulfilled. Will you experience the discrepancy discussed?
You will say “Wow, how difficult...”, and I will answer: “Who said that designing and maintaining management systems is easy?”
Okay, could there be a situation here where the discrepancy being discussed would be potential? Certainly. For example, an organization is preparing for a certification audit and studying the experience of other organizations in passing it. And she understands that she has a risk of non-conformity due to the fact that not all personnel are familiar with the quality policy. This is followed by an analysis of the system, why it allows this situation and the development of solutions that would change the system. These decisions will not differ from the above, the only difference is in the moment of action: CD - when the non-conformity is fixed, PD - when it is still at the stage of risk, opportunity.
Now let's look at the situation with the claims.
The presence of complaints for one type of product is our “past experience”, which we must use when analyzing possible inconsistencies. And, if indeed there is a real threat of receiving similar claims for another type of product, then the production and production management activities of these products should be analyzed in order to understand the reasons why these inconsistencies may arise. And change activities so that they do not arise.
Another thing is that finding the “reasons for complaints” can be difficult. Let me remind you that the reasons must lie in the system.
Maya asks: “Can we see examples of potential inconsistencies?” Of course you can. It is obvious that before a certification audit, any non-compliance of the QMS with the requirements of the standard is potential.
Or like this. The entrepreneur organized a taxi fleet by purchasing new cars. During the first months of operation, there was not a single vehicle that left the route due to a technical malfunction, i.e. there were no services that became non-compliant for this reason. But it is obvious that as the park operates, the risk will increase. And actions to reduce this risk must be taken before gatherings begin.
Summary:
- preventive action eliminates the cause and is therefore always aimed at changing the system (for us - QMS),
- PD are similar to CD - the difference is in the moment of decision making,
- training, familiarization, bonuses (de-bonuses), etc. – are not PD and CD, because do not change the system.
On what basis are they made?
As a result of inventories, audits, surveys and expert assessments of both the actual and expected state of the enterprise, one way or another, inconsistencies with plans, programs and other documentation and rules of enterprises may arise. In addition, QMS tools QMS mechanisms can also be used to identify and predict potential threats and hazards. Depending on the nature of these nonconformities, whether they are potential or already detected, either preventive or corrective actions are applied to them.
Key sources of information may be audit results in the following areas:
- Customer satisfaction with product quality (both external and internal);
- Internal audits (based on the quality plan);
- Secondary and key processes;
- Analysis of finished products.
The development of PD and CD procedures is a necessary measure in the activities of every organization. But especially one where there are frequent cases of equipment failure, all kinds of malfunctions, non-compliance with requirements, the emergence of possible dangers (major accidents with damage to property, the environment, the health of workers, business, and so on).
These failures are the consequences of imperfect work organization: outdated technology, human factor, natural influences, as well as many other factors. The life of any organization cannot do without such excesses, but some easily overcome the invisible line, while others suffer catastrophic losses. And the reason is the reasonableness and adequacy of preventive and corrective actions, which also require the competent use of financial, labor and time resources.
Working with threats, taking place in conditions of high uncertainty, has the nature of a probabilistic discipline. This discipline is called risk management. It is this area of activity that includes the prevention of nonconformities, which is one of the requirements of the ISO 9000 series.
Risk management includes a certain sequence of actions:
Corrective and preventive actions are measurable. Their effectiveness can be assessed using the formulas:
P i =R 0 -R i(1)
Ei= C 0 i— C i(2)
where Pi is the effectiveness of the i-th event, Ro is the risk before the i-th event, Ri is the risk after the i-th event, Ei is the effectiveness of the i-th event, Coi is losses due to undesirable consequences before the i-th event activities, Ci are losses due to undesirable consequences after the implementation of the i-th activity.
The effectiveness of the procedures increases as the values of the Ei and Pi indicators increase.
How to manage corrective actions?
The ISO 9001:2008 standard provides two types of directions in which it is necessary to move if inconsistencies are discovered at the enterprise. The first is correction, the second is corrective action.
At first glance, there seems to be no difference between them. However, this is not quite true. Correction is an event that is aimed at eliminating the emerging deviation. Corrective actions are aimed at eliminating the causes of this discrepancy.

The first step of this procedure requires identifying the sources of data on the identified nonconformities. At the same time, it is also worthwhile to provide for the presence of mechanisms with the help of which data on current deviations can be obtained.

At the second stage, the time comes to determine the need for corrective actions based on the significance of the consequences of the identified inconsistencies. At the same time, the cost of eliminating the cause of the deviation is calculated and the likelihood of its reoccurrence is determined.
The third stage involves analyzing the causes of these failures using group methods, for example, brainstorming, the “five whys” method, the precedent method, constructing an Ishikawa diagram and other methods.
The fourth stage is the time for specific actions. At this step, measures are developed and implemented to eliminate the causes of nonconformities. They usually improve the functioning of the organization as a whole and are carried out in relation to:

The next - fifth stage - determines the rules for correlating various deviations with levels of improvement in the enterprise's activities. It is worth keeping in mind that corrective actions can affect all levels of improvement - operational, medium-term and long-term. Operational improvements occur within the framework of individual works, medium-term ones have an impact on the processes themselves or the infrastructure of the enterprise, and long-term ones have an impact on all processes occurring in the organization, as well as its infrastructure and production area.

Final stage
Finally, the organization carries out procedures for analyzing the effectiveness and efficiency of the corrective actions taken - in order to clearly confirm their benefits and the justification of their costs. The main criterion for the effectiveness of corrective actions is the absence of repeated deviations from the standard.
Development of a methodology for managing preventive actions.
The method of performing preventive actions is in many ways similar to the procedure for implementing corrective actions,

but the difference between them is very significant. It lies in the methods of obtaining initial data on possible deviations and the specifics of measures to eliminate them. Preventive actions are a more complex procedure compared to CA, since it requires the presence of certain methods for managing probabilistic events that may or may not occur at the enterprise. This is working with risks that are always present in any activity, but are not always realized by its participants. Taking this into account, one of the highest functions of management is precisely to work with risks. Unfortunately, the nature of preventive actions is not always obvious, so there are a number of events that are often mistakenly taken as warnings:

To distinguish a warning action from its logical counterpart, you need to pay attention to the following factors:

The first stage of preventive action is to identify sources of information about possible future nonconformities. The list of these sources is similar to the list of sources of information on the design documentation.

Along with corrective actions, preventive actions can also address various levels of improvement in the enterprise - operational, medium-term and long-term.
The next, second stage is associated with the rules for correlating possible deviations with levels of improvement in the organization’s activities. If threats are identified, then after determining the appropriate management level, a person is appointed who is responsible for implementing preventive actions. The level of management corresponding to a possible threat is understood as a person in charge of an employee (or group of employees) who has the ability to eliminate the root cause of the threat.
Preventive actions are also carried out in several stages. These include:

How is the effectiveness of preventive actions calculated?
With the help of regular monitoring by the responsible person of objects suspected of frequent non-compliance with standards. The performance criterion is the absence of deviations in the area subject to preventive measures. The difference between PD and CA mainly lies not in general terms, but in the details of actions determined by the selected risk management methods.
Conclusions.
Corrective and preventive actions at the enterprise - if they are documented and well thought out - are the most important area of activity that improves both the functioning of the quality management system at the enterprise and the development of the entire organization as a whole. Thanks to methods for analyzing the causes of deviations, risk management, measures to prevent deviations taking into account measured risks and many others, high competitiveness of products is achieved, the company’s reputation and its reliability in the eyes of both partners and investors increases. When implementing a quality management system at an enterprise in accordance with ISO 9001:2008, you should carefully consider the development of design documentation and design documentation and their mandatory documentation - then the adaptation of the QMS will be easier and more effective.
5.2 Scheme for carrying out preventive actions in projects
Preventive actions in projects are carried out at the operational level of management. The period for carrying out preventive actions corresponds to the established periods for monitoring the status of the project work - weekly project meetings and project status meetings.
If inconsistencies may arise, the project manager indicates them in the minutes of the weekly or status meeting in the “Potential problems” column. If it is necessary to take preventive actions, these actions are indicated in the “Decisions taken” column. The effectiveness of preventive actions is assessed during subsequent weekly or status meetings on the project.
The effectiveness of preventive actions in a project is assessed at the end of the project. The evaluation is carried out by the executive director based on the achievement of project targets.
5.3 Scheme for carrying out preventive actions in the quality management system
Preventive actions in the quality management system are carried out at the tactical and strategic levels of management. The period for carrying out preventive actions in the quality system corresponds to the periods of internal audit and analysis of the QMS by management - once every six months.
A self-assessment of the company's activities is carried out annually. The results of self-assessment are used to carry out preventive actions together with data from internal audits and analysis of the QMS performance for the year.